Recognised know-how in the health sector
PRINCE MEDICAL, certified NF EN ISO 13485, designs and manufactures single-use medical devices for Gastroenterology, Gynaecology and Medically Assisted Reproduction.
With nearly 30 years of expertise in the healthcare market, the company can rely on technical know-how in the extrusion of thermoplastics and polymers, and has state-of-the-art equipment to meet the needs of markets with demanding applications.
Perfect control of all industrial processes (moulding, extrusion, production of latex and silicone balloons, assembly of plastic material and steel components, laser cutting, ultrasonic and laser welding, marking, packaging, sterilisation, etc.) is a guarantee of the high level of quality of PRINCE MEDICAL products.
Key figures
Our solutions
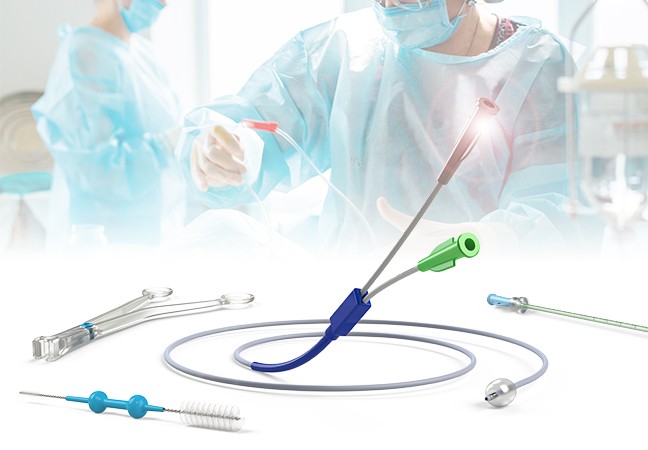
PRINCE MEDICAL offers a wide range of sterile Class IIb, Class IIa, Class I sterile and non-sterile medical devices, mainly intended for:
- Gastroenterology: ballons catheters, injection needles, cleaning brushes for endoscopes, etc.
- Gynaecology: gynaecological forceps (Cheron forceps, Pozzi forceps, etc.), speculums, histological swabbing probes, etc.
- Medically Assisted Reproduction MAR: embryo transfer catheters, intrauterine insemination catheter, puncture needle of oocytes, etc.
PRINCE MEDICAL also manufactures medical devices on behalf of its customers for use in urodynamics, anaesthesia, oxygen therapy, as well as external lines for infusion and injection of contrast media in radiology.
PRINCE MEDICAL is a subsidiary of the international OMERIN Group and benefits from the dynamism of its 1,700 employees.