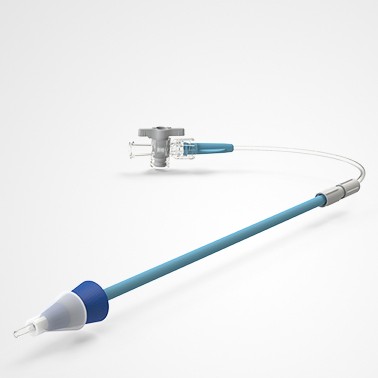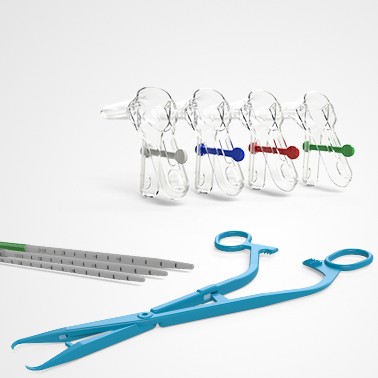

PRINCE MEDICAL, certified NF EN ISO 13485, designs and manufactures a wide range of medical devices (sterile Class IIb, Class IIa, Class I sterile and non-sterile) for Gynaecology: gynaecological forceps (Cheron forceps, Pozzi forceps, etc.), speculums, histological swabbing probes, etc. Our PM-CARE® medical devices are used for sampling, smear and biopsy, intra-uterine device insertion and removal, hysterography and hysterosonography, functional exploration. With nearly 30 years of expertise in the healthcare market, the company can rely on technical know-how in the extrusion of thermoplastics and polymers, and has state-of-the-art equipment to meet the needs of markets with demanding applications. The company manufactures and packages its products in a controlled environment and has numerous grey and ISO7 and ISO8 classified clean rooms, guaranteeing a high level of product safety.
Medical devices PM
Other Healthcare solutions from the OMERIN Group
Downloads
Gynaecology
INFORMATION

Réservé aux professionnels de santé
Le site www.prince-medical.com permet aux professionnels de santé spécialisés dans les domaines de la Gastro-entérologie, Gynécologie, Procréation Médicalement Assistée de bénéficier d'informations utiles à leur pratique quotidienne.
Reserved for health professionals
The website www.prince-medical.com allows health professionals specialized in the sectors of Gastroenterology, Gynecology, Medically Assisted Reproduction to benefit from information useful to their daily practice.